Understanding of magic questions for chemistry quiz in competition has important value. For that reason, this article is arranged for students from a different stream. Due to changes in exam patterns and present tough situations, we provide all the easy steps to learn in mind. So, we present such a lucid and interesting article for our visitors after a long period.
Finally, we expect you to encourage us to go forward for our new generation. Wherever, benefit comes through our little contribution. Besides, your few interests and our constant labour can send you to the high sky in a new era. For the best experience in mastering chemistry without cost, visit our site with a smile.
So far, magic questions for chemistry quizzes are needed for you to crack the top exams. Afterwards, it might be UPSC CSE, NEET, JEE, GRE, SAT, PTE, etc excellence. Now let us write some interesting GK questions with answers for the top jobs. At the same time, you can prepare for exams and test preparation too. Thus, there can be higher education exams in the future also. These are from magic questions for chemistry quiz which helps lots. Hence, you cannot believe this unexpected thing at all.
The High Magic Questions for Chemistry Quiz Now
Herbicide
Anesthetic
VAN HELMONT
Antiseptics
Fungicides
A Substance
Condensation
00C (Zero degrees centigrade)
Amorphous
Hydrogen Sulphide Gas (H2S)
Without doubt, Silver metal has a greater number of a free-electron than copper
HENRY CAVENDISH
Diffusion
Element
Atoms in solids are tight-packed due to the strong force of attraction among atoms
An element
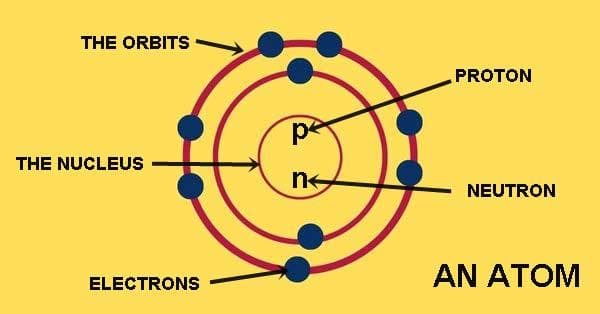
Protons
Due to the same number of Protons and electrons

States of Matter in Magic Quiz Questions Sure Now
Due to temperature or pressure
Condensation
JOSEPH PRIESTLEY
The Periodic Table
Protons
Metalloids (e.g., Boron)
Noble Gases (Helium, Neon, etc…)
Electrons (Surround the nucleus)
Cuprum
Oxygen (65%), Carbon (18%), and Hydrogen (10%)
Helium, Neon, Argon, Krypton, Xenon, and Radon
Freezing point
Extreme right of the periodic table (Same Group i.e., similar properties)
Atomicity
Concept of Valency Now Thrill in Chemistry Quiz
Valency
Variable Valency (e.g., Cu1+, Cu2+)
What Is the Name of the Element Having 3 Variable Valency?
Variable valency is seen in metals such as iron, mercury, and copper. This variable valency found in transition elements. For example, iron shows both two and three valencies. The valency of 2 like ferrous sulphate (FeSO₄) and a valency of 3 like ferric chloride (FeCl₃).
Positive valencies [K1+, Na1+, Ca2+, Mg2+, Zn2+, Al3+]
Group of atoms of elements [e.g., NO3, OH, SO3, SO4, CO3, PO4]
Negative valencies [Cl1-, Br1-, I1-, O2-, S2- and NO3 1-, OH1-, SO3 2-, SO42-, CO32-, PO43-]
Noble Gases
Protons [positive charged] and neutrons [no charged]
Left of the periodic table (for Metallic) and right of the periodic table (for Non-Metallic)
FAQs
What are the 3 most important uses of carbon dioxide?
Three main uses of carbon dioxide (CO2) are-
- Plants use carbon dioxide with green pigment (CH2O) during photosynthesis for making food.
- It used for making cold drinks. For example- coca cola, soda water etc.
- It uses for preparation of washing soda (Na2CO3· 10H2O) or soda ash.
Why is noble gas called inert gas?
Noble gas shows great steadiness and so tremendously low reaction rates. Every noble gas except helium (He) has eight electrons in its outermost orbit. As a result, octet (eight-electron arrangement) is fulfil. So, there is no tendency to react. So, it is called inert gas.
What is the reason for variable valency?
Electronic configurations of a element is the main cause of variable valency. It is due to the position of electrons changing its shells. Certain electrons cause additional electron loss. So far, electrons jump between shells due to empty orbitals in the same subshell.
What Is the Benefit of Learning Basic Questions for Chemistry Quiz?
Chemistry helps us to make agriculture, medicine, and other products. Hence, knowledge of chemistry is valuable for everyone. So, several chemical compounds are useful in making our fertilizers, insecticides, fungicides. Later, it is also used in making herbicides. Thus, knowledge of chemical compounds gives you extra bonus points. And so, based on that we have presented some questions for the chemistry quiz. Right now, this knowledge will give us an unbelievable advantage. We are thankful to MENDELEEF, MOSLEY, RUTHERFORD, and many others for their contribution. We learn the study of substances and their composition from our respective scientists. Thus, we thank the chemists and their discoveries. This is how questions for chemistry quiz are presented here.
Ultimate Awesome Reading in Rich questions for chemistry quiz
The questions we provide for chemistry quizzes serve several important purposes. So, we design the questions in such a way to understand our basic concepts. As a result, we help the reader’s knowledge to be powerful. Do not forget to give important information. And so, by engaging with these questions, we deepen our chemical understanding. At the same time, we get to know the properties of materials and various other aspects. Finally, quizzes encourage active participation and critical thinking. In this way, chemistry enhances our learning experience.
Furthermore, we are waiting for your opinion please. Again, we hope you have revised some of the questions for chemistry quiz very well. Please let me know if you need to update this article yet. So, do not forget to comment whether it is good or bad.